REPRIEVE Facts
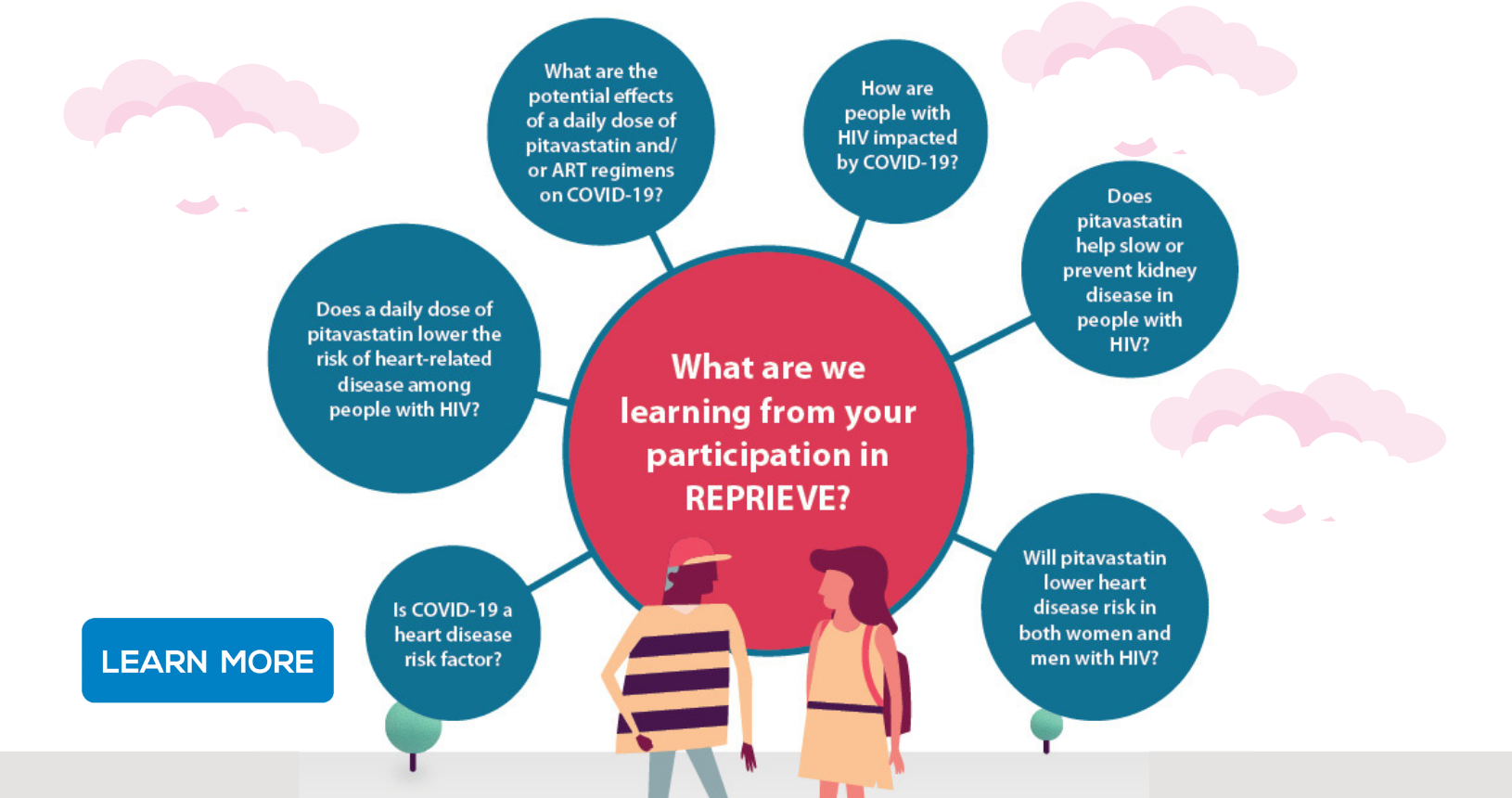
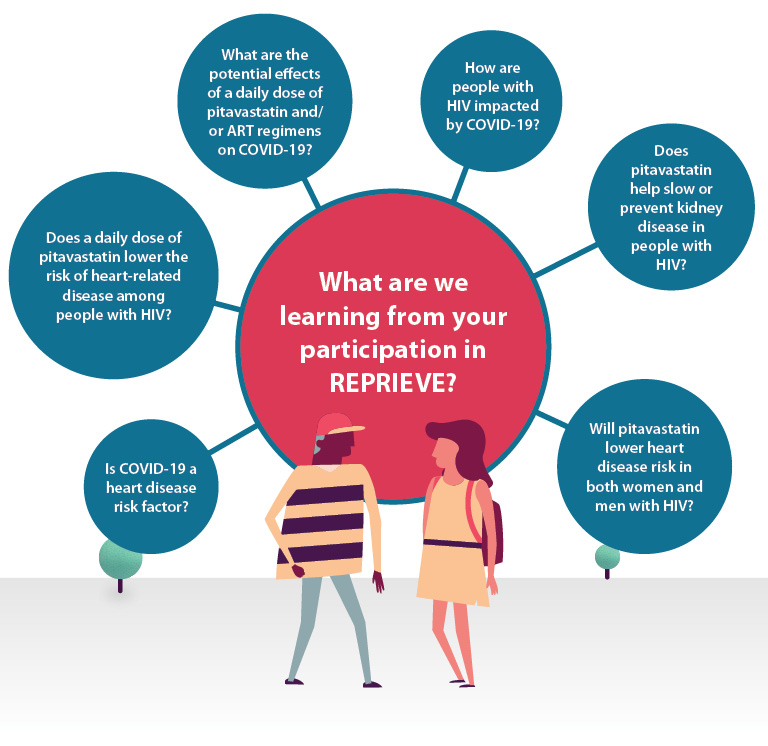
REPRIEVE evaluated if a statin medication (pitavastatin calcium) is effective to prevent heart disease among people with HIV.
REPRIEVE is the largest randomized trial to date in HIV and results from REPRIEVE are helping clinical researchers and clinical care providers to develop heart disease prevention and treatment guidelines, specifically for people with HIV.
Number of Participants
7769
Percentage of Women
32%
Mean Age
50
Mean Years Living with HIV
13
Manuscripts Published
38
Over 7500 Participants Have Joined REPRIEVE!
Here are two of their stories.

Marcus Conway,
College Park, Maryland, USA
"The best thing about participating in a clinical trial is learning more about a disease or illness. I believe that my participation in REPRIEVE has helped me to become more knowledgeable about HIV and heart disease prevention. I am hopeful that the results of REPRIEVE show that heart disease events can be prevented with the use of a statin medication."

Ernane Pinho,
Pavuna, Rio de Janeiro
“For me, REPRIEVE is important to monitor and control my cholesterol rates, and for the community living with HIV it is another form of care and prevention for good health.”
REPRIEVE is a global study
There are over 100 clinical sites in 12 countries around the world
Supporting Organizations