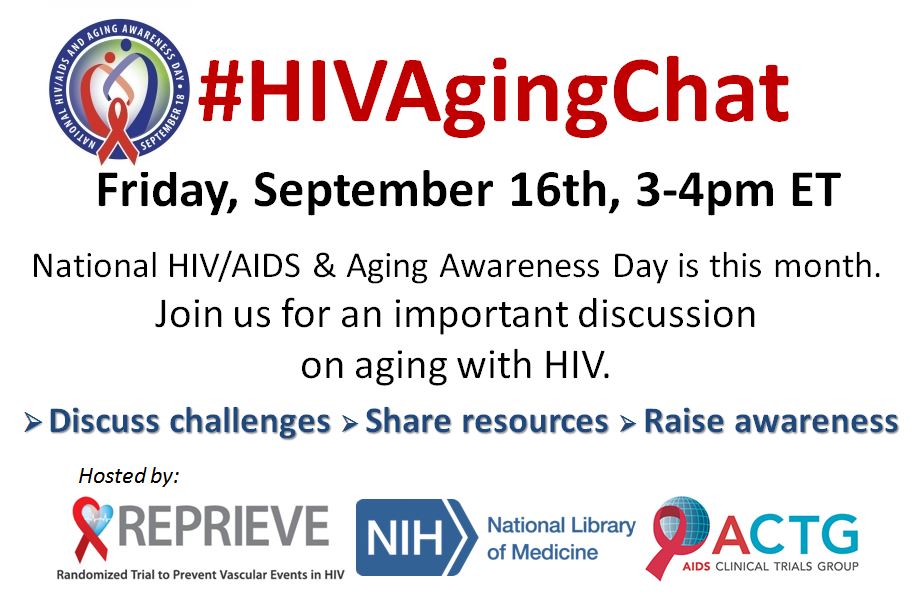June 2013 Letter of Acceptance from NIH-NHLBI
The NHLBI reviews the letter from REPRIEVE investigators requesting permission to submit a new clinical trial grant entitled “Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE).” The NHLBI indicates it will accept the application for review.
September 2013 Submission of U01 cooperative agreement grant
Grant application for the REPRIEVE Clinical Coordinating Center and Data Coordinating Center to conduct REPRIEVE is submitted to the NIH NHLBI.
December 26, 2013 Favorable scientific review of U01 cooperative grant
The U01 grant application seeking funding for REPRIEVE undergoes a highly favorable scientific peer review by the NIH NHLBI and NIAID. Given the favorable review, the study team decides to move forward with addressing the comments and proceed with further protocol development.
September 2014 The REPRIEVE DSMB inaugural meeting takes place
The inaugural meeting of the REPRIEVE Data Safety Monitoring Board (DSMB) takes place. The DSMB was established by the NHLBI to monitor data and oversee patient safety in REPRIEVE. The DSMB is comprised of members including senior experts in cardiovascular disease, HIV medicine, statistics, and ethics, as well as a representative from the HIV community. The DSMB is appointed by the Director of NHLBI and convenes approximately twice a year. The DSMB is advisory to both the NHLBI and NIAID, as both Institutes are supporting REPRIEVE.
October 27, 2014 1st Press Release About REPRIEVE
Massachusetts General Hospital (where the REPRIEVE Coordinating Center is based) distributes a
press release to announce the official start of REPRIEVE activities.
December 14, 2014 Protocol is distributed to the field
Sites can begin to prepare the protocol for local regulatory approvals and can move toward site activation and ultimately enrollment of participants in REPRIEVE.
February 2015 First REPRIEVE Investigator’s Meeting takes place in Seattle, WA
The first Investigator’s Meeting takes place after CROI 2015. The meeting focused on timelines and study requirements as well as training to conduct the protocol.
March 9, 2015 First REPRIEVE Site Newsletter is distributed
Site Newsletters are created by the Clinical Coordinating Center team and sent via email monthly. The newsletters include important updates for REPRIEVE site staff including helpful tips, FAQs, and features of clinical site teams. Click
here to read our newsletters.
March 24, 2015 Participant recruitment begins
REPRIEVE begins screening people with HIV who are at low to moderate risk for developing cardiovascular disease. Clinical Site 31788 Alabama CRS is the first site to become activated and the first site to enroll a participant.
April 15, 2015 2nd Press Release to Announce the Launch of REPRIEVE
NIH distributes a press release to announce the launch of REPRIEVE activities.
November 9, 2015 Recruitment Champion Initiative is Launched
Dr. Markella Zanni, REPRIEVE Co-Investigator, begins phone interviews with Recruitment Champions at over 50 REPRIEVE clinical sites to better understand recruitment practices, barriers and best practices. Information gathered from this initiative was shared with all clinical site teams in REPRIEVE.
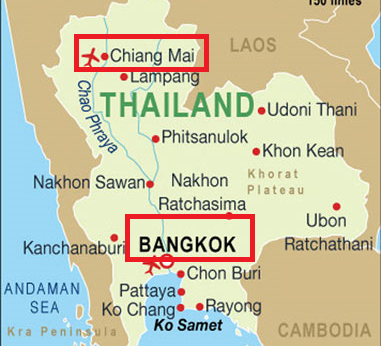
September 17, 2015First International Sites are Activated
Sites 31784 Chiang Mai and 31802 Thai Red Cross AIDS in Thailand open to enroll participants. These are the first 2 clinical sites outside the US to enroll participants.
December 14, 2015REPRIEVE video launched
The REPRIEVE video is released and utilized as a tool to educate the community about the importance of the REPRIEVE trial. Sites are encouraged to share the video with participants, community advisory boards, clinicians, and local media. The video can be viewed
here.
December 24, 2015 Review of ancillary substudy proposals
The first review of ancillary substudies takes place resulting in the eventual approval of 6 ancillary substudies and objectives. Click
here for more details on these interesting studies
February 17, 2016 REPRIEVE Hosts first Twitter Chat
To recognize Heart Month, the REPRIEVE team hosts its first ever Twitter chat about HIV and heart disease. 32 organizations joined this event including NHLBI, NIAID, AIDS Action, The Well Project, and the ACTG Network. #hivheartchat
February 22, 2016 Over 1000 participants enrolled
The University of Washington AIDS CRS site enrolled the 1000th participant, REPRIEVE leadership is pleased that this major accrual milestone has been met.
April 4, 2016 Follow YOUR Heart Campaign Launched
Dr. Sara Looby and Dr. Markella Zanni of Massachusetts General Hospital launch Follow YOUR Heart, a campaign designed to educate women living with HIV about heart disease and HIV and the importance of women’s participation in clinical research studies like REPRIEVE. This launch included a website, video and the My Heart Matters blog. Funding from NIAID to support this campaign in addition to the evaluation of sex-specific mechanisms of cardiovascular disease risk and risk reduction in the context of the REPRIEVE trial helped to carry out this important campaign.
April 20, 2016 First Participant Newsletter
The first participant newsletter is released and features short bios of current REPRIEVE participants, FAQs, and important study updates. The REPRIEVE Participant Newsletter is released annually in April.
Click here to read all the newsletters.
August 23, 2016 REPRIEVE joins Ryan White Annual Conference
REPRIEVE joins other HIV advocacy groups and organizations at the Annual Ryan White Conference. Materials about REPRIEVE are available to make community members aware of this landmark trial.
September 13, 2016 Barbra Streisand and Dr. Anthony Fauci support REPRIEVE
The article “A Reprieve for Women: Embracing Inclusive Scientific Research”, is published on the Health Affairs Blog. The article, co-authored by Barbra Streisand and Dr. Anthony Fauci underscores REPRIEVE as a unique, well-designed study that takes sex and gender into account from recruitment to study data analysis. Read the article
here.
September 16, 2016 REPRIEVE Hosts 2nd Twitter Chat on HIV and Aging
In observance of National HIV/AIDS & Aging Awareness Day, REPRIEVE partnered with the AIDS Clinical Trial Network, the National Library of Medicine, and ACRIA to host an HIV and Aging Twitter Chat (#HIVAgingChat). The purpose of the chat was to raise awareness about aging with HIV, discuss the challenges associated with it and to provide resources for people living with HIV.
October 10, 2016 Over 2000 participants enrolled
The Northwestern University CRS enrolled the 2000th participant in REPRIEVE! Dr. Carl Dieffenbach, Director of the Division of AIDS, NIAID creates a special thank you message to clinical sites about reaching this major milestone.
November 11, 2016 100 clinical sites activated to enroll participants
An amazing accomplishment of activating 100 clinical sites in over 10 countries to enroll participants in REPRIEVE. This accomplishment could not have been reached without the combined efforts of the Site Activation Team and the Site Selection and Performance Committee chaired by Dr. Carl Fichtenbaum.
February 17, 2017 Volunteers share experience about clinical research and REPRIEVE
Dr. Carl Dieffenbach, Director of the Division of AIDS, NIAID interviews volunteers at the University of Washington AIDS CRS about their experiences participating in clinical research including REPRIEVE. Watch the video
here.
March 18, 2017 Follow YOUR Heart campaign is published in HIV Clinical Trials.
The methods of the Follow YOUR Heart campaign led by Dr. Sara Looby and Dr. Markella Zanni are published. Follow YOUR Heart: Development of an Evidence-Based Campaign Empowering Older Women with HIV to Participate in a Large-Scale Cardiovascular Disease Prevention Trial. This campaign is designed to educate women about the importance of women’s participation in clinical research studies like REPRIEVE.
Read more
March 22, 2017 Over 3000 participants enrolled!
Congratulations to the Thai Red Cross team for enrolling the 3000th participant.
August 7, 2017 Over 4000 participants enrolled!
REPRIEVE’s enrollment hits another major milestone, over 4000 participants are now enrolled. Congratulations to the University of Cincinnati for enrolling the 4000th participant.
September 11, 2017 REPRIEVE Outreach Toolkit is available
The REPRIEVE Clinical Coordinating Center partnered with the NIAID Communications team to compile helpful outreach resources. This “Outreach Toolkit” is an online webpage with resources to assist sites looking to improve outreach, increase enrollment, engage with the community, connect with local media outlets and raise awareness about HIV and cardiovascular disease.
September 26, 2017 First REPRIEVE Community Advisory Board Meeting held
While REPRIEVE has greatly benefitted from the ACTG CSS representatives, since both ACTG and non-ACTG sites are included a CAB was established to represent the trial. Objectives of the REPRIEVE CAB are to incorporate the voice of the participant into trial activities, gather diverse perspectives to improve participant initiatives and engagement, provide a forum for enthusiastic participants to share their input, liaise with trial leaders, and interact with other CAB members. Meet our CAB members
here.
November 27, 2017 Over 5000 participants enrolled!
REPRIEVE’s enrollment hits another major milestone, over 5000 participants are now enrolled. Congratulations to the Family Clinical Research Unit team for enrolling the 5000th participant.
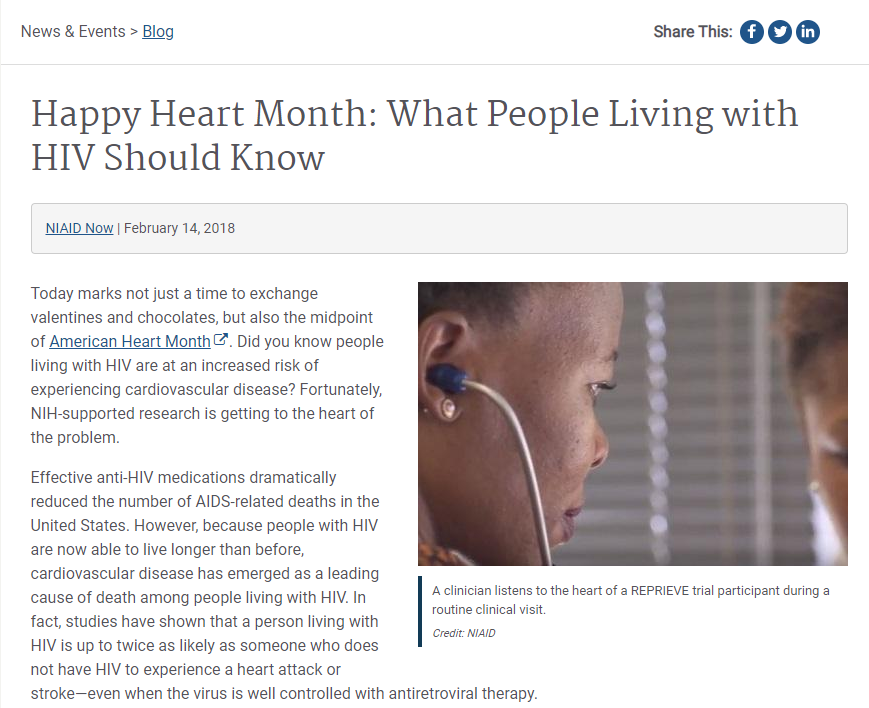
February 14, 2018 REPRIEVE is featured on NIAID blog
NIAID features REPRIEVE on their blog. The post features a summary of REPRIEVE and its importance for those living with HIV. Listen
here.
March 12, 2018 Over 6000 participants enrolled!
REPRIEVE’s enrollment hits another major milestone, over 6000 participants are now enrolled and getting closer to the accrual target of 7500 participants. Congratulations to the Washington University Therapeutics team for enrolling the 6000th participant.
June 2018 2018 Annual ACTG Network Meeting
Approximately 300 people, including REPRIEVE study team members and participants, attended the annual REPRIEVE Session at the 2018 ACTG Meeting. The session included a presentation by Dr. Gail Pearson from NHBLI as well as REPRIEVE’s Team Cardiologist and Co-PI, Dr. Pam Douglas.
July 15, 2018 Retention Champion Initiative Launched
Members of the REPRIEVE Clinical Coordinating Center begin phone interviews with Retention Champions at over 50 REPRIEVE clinical sites to better understand retention practices and common barriers. Information gathered from this initiative was shared with all clinical site teams in REPRIEVE and is available in the Retention Toolkit. Click here to access the Retention Toolkit.
September 6, 2018 Over 7000 participants enrolled
REPRIEVE’s enrollment hits another major milestone, over 7000 participants are now enrolled. Congratulations to the Whitman Walker team in Washington DC, USA for enrolling the 7000th participant.
7500th participant enrolled
REPRIEVE leadership celebrates reaching target accrual of 7500 participants. Congratulations to the University of Southern California for enrolling the 7500th participant!
February 25, 2019 REPRIEVE Ambassador Program Launched
REPRIEVE Clinical Coordinating Center launches the REPRIEVE Ambassador Program with a visit to The Miriam Hospital in Providence, RI. Over the course of the trial, members of the REPRIEVE CCC will be making an effort to visit a large number of REPRIEVE sites to ensure they feel supported in their trial efforts.
March 6, 2019 REPRIEVE Investigator’s Meeting
Investigator’s Meeting is held adjacent to the Conference on Retroviruses and Opportunistic Infections meeting in Seattle, WA. The mood at the meeting was celebratory due to the recent completion of enrollment. Co-Investigator Carl Fichtenbaum writes and preforms an original song in recognition of this major milestone. Watch the video
here.
March 25, 2019 REPRIEVE closes to enrollment
Leadership from the NIH, including Dr. Anthony S. Fauci, Director of NIAID, and Dr. Gary H Gibbons, Director of NHLBI put together a thank you video for REPRIEVE participants and sites in recognition of this incredible milestone. Watch the video
here.
July 10, 2020 REPRIEVE baseline data published in The Journal of Infectious Diseases
Six REPRIEVE manuscripts, plus an introduction piece, highlighting key baseline data were published as a supplement in The Journal of Infectious Diseases! The suite of manuscripts highlight the impact and prevalence of critical comorbidities among a global cohort of people with HIV. Read the full set of articles online
here.
July 2020 REPRIEVE reaches 10 manuscripts published
Ten REPRIEVE manuscripts describing the study's design and recruitment strategies and analyzing baseline data have been published!
September 10, 2020 REPRIEVE receives supplemental funding from NHLBI to study COVID-19 in the global REPRIEVE cohort
The NIH was excited to leverage our robust trial infrastructure to achieve these aims and further assess the important question of statin effects on COVID-19 disease severity. Funds from the NIH were awarded after a very exhaustive process in which our collective work to maintain trial integrity was recognized, and the importance of our large, global cohort of PWH acknowledged. In addition to being the largest trial to evaluate a strategy to prevent cardiovascular disease among PWH, REPRIEVE may also be the largest study of COVID-19 among PWH.
Learn more
here.
June 17, 2021 REPRIEVE hosts its first Community Forum
The REPRIEVE team held a virtual Community Forum as part of the ACTG Annual Network Meeting. This Forum was an opportunity to share baseline trial findings and discuss trial progress. This event was attended by more than 300 members of the community!
July 2022 REPRIEVE publishes its 20th manuscript
REPRIEVE reaches 20 published manuscripts! The 20th publication, Diet in a global cohort of adults with HIV at Low to Moderate Traditional Cardiovascular Disease Risk, is available to read online
here.
November 2022 REPRIEVE publishes its 25th manuscript
REPRIEVE reaches 25 published manuscripts! The 25th publication, The importance of methods for site performance evaluation in REPRIEVE, a longitudinal, global, multicenter trial, is available to read online
here.
March 2023 REPRIEVE Data and Safety Monitoring Board Meeting
A planned interim analysis of data from REPRIEVE finds that participants who took pitavastatin calcium, a daily statin, lowered their risk of major adverse cardiovascular events by 35% compared with those receiving a placebo. Adverse drug events observed in the study were like those in the general population taking statin therapy. The interim analysis was sufficiently compelling that the study’s independent Data Safety and Monitoring Board (DSMB) recommended it be stopped early given adequate evidence of efficacy.
April 2023 REPRIEVE Announces Study Closure for Efficacy
REPRIEVE and the NIH announce that the trial is closing for efficacy. Read the full NIH press release about the DSMB's decision
here.
June 13, 2023 REPRIEVE Presents Topline Results at ACTG
REPRIEVE presents topline results from the trial for the first time as the focus of the opening plenary for the annual ACTG Network Meeting.
June 26, 2023 REPRIEVE publishes its 30th manuscript
REPRIEVE reaches 30 published manuscripts! The 30th publication, Associations of Muscle Density and Area with Coronary Artery Plaque and Physical Function, is available to read online
here.
November 11, 2023 Results of the REPRIEVE Mechanistic Substudy Are Presented at AHA 2023
The results of the REPRIEVE Mechanistic Substudy are presented at the American Heart Association 2023 Conference in Philadelphia, PA.
Find news coverage of the presentation
here.
November 21, 2023 British HIV Association Rapid Guidance is Released
The British HIV Association releases rapid guidance on the use of statins to prevent heart disease in people living with HIV
Read the guidance
here.
May 2, 2024 Final REPRIEVE Results Are Published in NEJM, the Trial's 40th Manuscript